noun Physics.
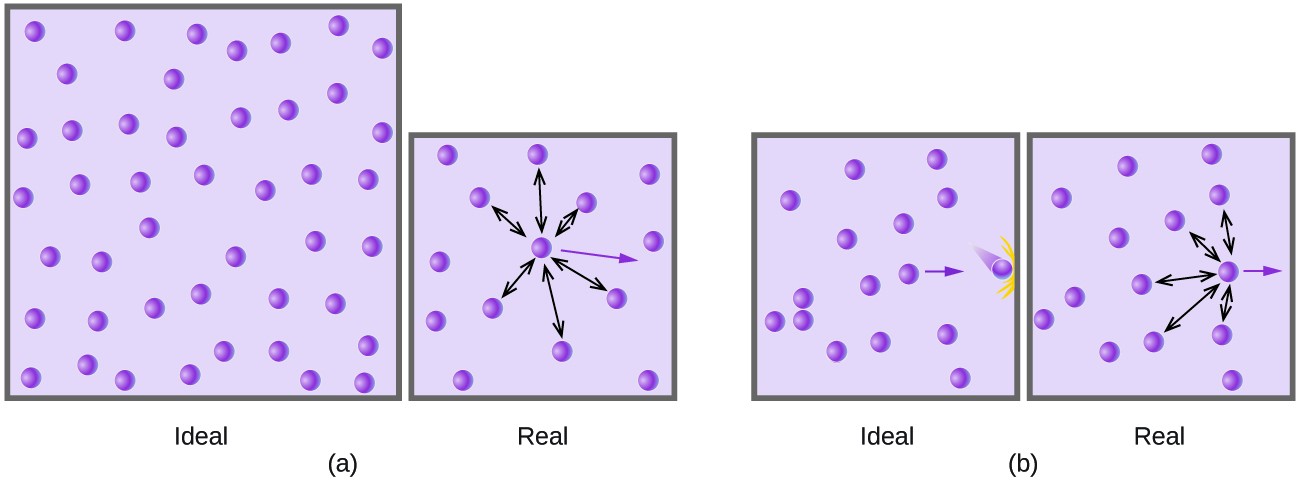
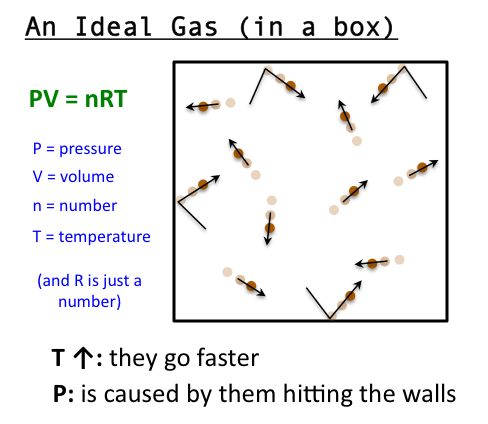
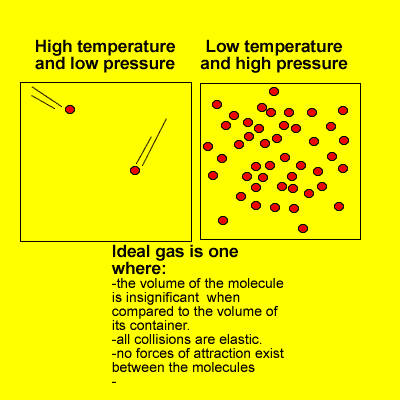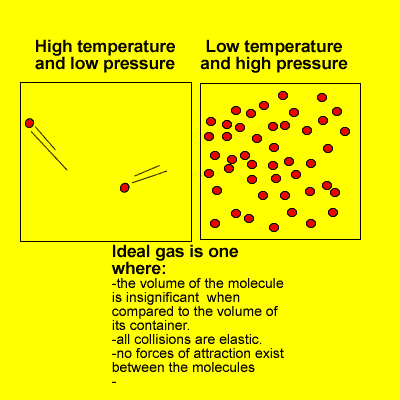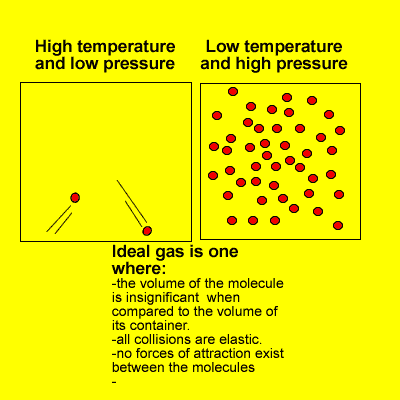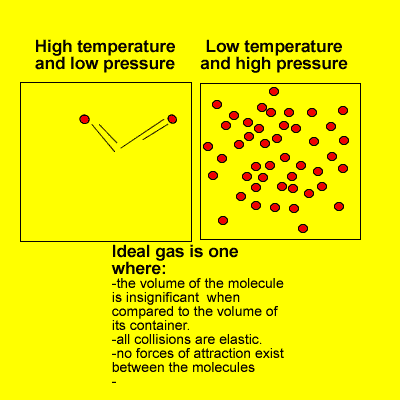
- a gas composed of molecules on which no forces act except upon collision with one another and with the walls of the container in which the gas is enclosed; a gas that obeys the ideal gas law.
noun
- a hypothetical gas which obeys Boyle’s law exactly at all temperatures and pressures, and which has internal energy that depends only upon the temperature. Measurements upon real gases are extrapolated to zero pressure to obtain results in agreement with theories relating to an ideal gas, especially in thermometryAlso called: perfect gas
n.
- A gas that, when kept at a constant temperature, would obey gas laws exactly. No known gas is an ideal gas.
- A hypothetical gas whose molecules bounce off each other (and the boundaries of their container) with perfect elasticity and have negligible size, and in which the intermolecular forces acting between molecules not in contact with each other are also negligible. Such a gas would obey the gas laws (such as Charles’s law and Boyle’s law) exactly at all temperatures and pressures. Most actual gases behave approximately as ideal gases, except at very low temperatures (when the potential energy of their intermolecular forces is high relative to the kinetic energy of the molecules and becomes significant), and under very high pressures (when the molecules are packed so close together that close-range intermolecular forces become significant).
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary