isotope [ahy-suh-tohp] ExamplesWord Origin noun Chemistry.
- any of two or more forms of a chemical element, having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic weights. There are 275 isotopes of the 81 stable elements, in addition to over 800 radioactive isotopes, and every element has known isotopic forms. Isotopes of a single element possess almost identical properties.
Origin of isotope 1910–15; iso- + -tope Greek tópos placeRelated formsi·so·top·ic [ahy-suh-top-ik] /ˌaɪ səˈtɒp ɪk/, adjectivei·so·top·i·cal·ly, adverb Examples from the Web for isotope Contemporary Examples of isotope
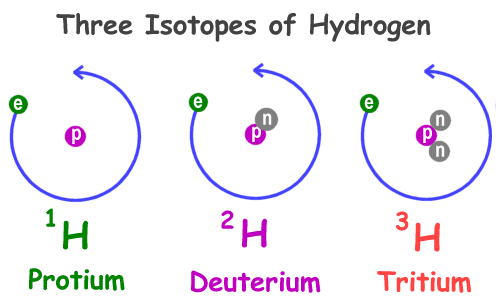
Deuterium is an isotope of hydrogen containing a proton and neutron in its nucleus, while normal hydrogen has only a proton.
Are Comets the Origin of Earth’s Oceans?
Matthew R. Francis
December 14, 2014
After eight years, traces of the isotope are expected to be extremely low.
Was Yasir Poisoned? Arafat’s Body Exhumed
Dan Ephron
November 27, 2012
Historical Examples of isotope
Why nail the “power metal” down to an isotope of gold with an atomic weight of 197?
Despoilers of the Golden Empire
Gordon Randall Garrett
An isotope is just a different variety of the ordinary kind of atom in each element.
John Blaine
There must be another one in either wing, for the isotope plant and the cartridge-case plant.
Henry Beam Piper
Well, heavy water is made of one atom of oxygen plus two atoms of deuterium, which is the first isotope of hydrogen.
John Blaine
British Dictionary definitions for isotope isotope noun
- one of two or more atoms with the same atomic number that contain different numbers of neutrons
Derived Formsisotopic (ˌaɪsəˈtɒpɪk), adjectiveisotopically, adverbisotopy (aɪˈsɒtəpɪ), nounWord Origin for isotope C20: from iso- + Greek topos place Word Origin and History for isotope n.
1913, literally “having the same place,” introduced by British chemist Frederick Soddy (1877-1956) on suggestion of Margaret Todd, from Greek isos “equal” (see iso-) + topos “place” (see topos); so called because despite the different atomic weights, the various forms of an element occupy the same place on the periodic table.
isotope in Medicine isotope [ī′sə-tōp′] n.
- One of two or more atoms having the same atomic number but different mass numbers.
Related formsi′so•top′ic (-tŏp′ĭk) adj. isotope in Science isotope [ī′sə-tōp′]
- One of two or more atoms that have the same atomic number (the same number of protons) but a different number of neutrons. Carbon 12, the most common form of carbon, has six protons and six neutrons, whereas carbon 14 has six protons and eight neutrons. Isotopes of a given element typically behave alike chemically. With the exception of hydrogen, elements found on Earth generally have the same number of protons and neutrons; heavier and lighter isotopes (with more or fewer neutrons) are often unstable and undergo radioactive decay.
isotope in Culture isotope [(eye-suh-tohp)]
In physics, different forms of the same element, with nuclei that have the same number of protons but different numbers of neutrons. Isotopes are distinguished from each other by giving the combined number of protons and neutrons in the nucleus. For example, uranium 235 is the isotope of uranium that has 235 protons and neutrons in its nucleus rather than the more commonly occurring 238. All elements have isotopes.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary