noun Chemistry.
- the type of chemical bond between atoms in a metallic element, formed by the valence electrons moving freely through the metal lattice.
noun
- chem the covalent bonding between atoms in metals, in which the valence electrons are free to move through the crystal
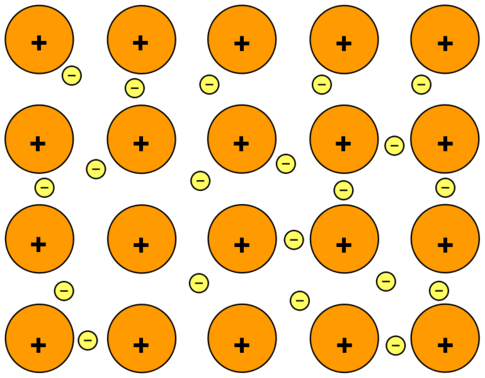
- The chemical bonding that holds the atoms of a metal together. Metallic bonds are formed from the attraction between mobile electrons and fixed, positively charged metallic atoms. Whereas most chemical bonds are localized between specific neighboring atoms, metallic bonds extend over the entire molecular structure.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary