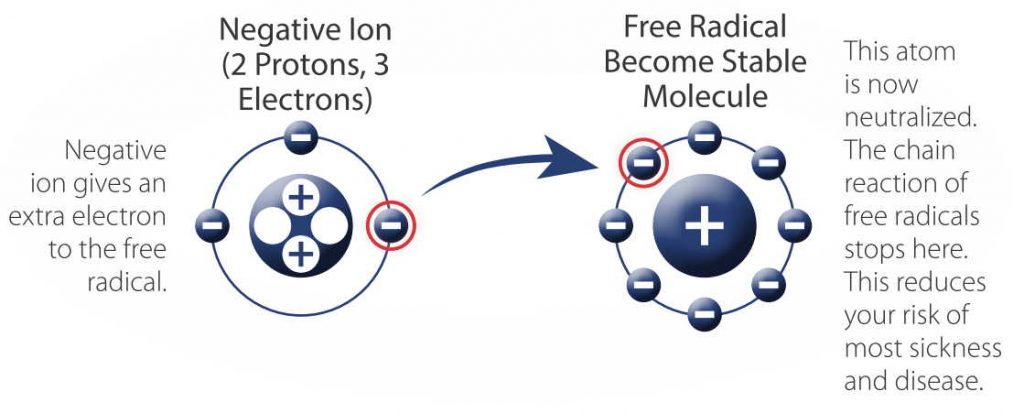
noun Physics, Chemistry.
- See under ion(def 1).
noun Physics, Chemistry.
- an electrically charged atom or group of atoms formed by the loss or gain of one or more electrons, as a cation (positive ion), which is created by electron loss and is attracted to the cathode in electrolysis, or as an anion (negative ion), which is created by an electron gain and is attracted to the anode. The valence of an ion is equal to the number of electrons lost or gained and is indicated by a plus sign for cations and a minus sign for anions, thus: Na+, Cl−, Ca++, S=.
- one of the electrically charged particles formed in a gas by electric discharge or the like.
noun
- an electrically charged atom or group of atoms formed by the loss or gain of one or more electronsSee also cation, anion
n.1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion, neuter present participle of ienai “go,” from PIE root *ei- “to go, to walk” (cf. Greek eimi “I go;” Latin ire “to go,” iter “a way;” Old Irish ethaim “I go;” Irish bothar “a road” (from *bou-itro- “cows’ way”), Gaulish eimu “we go,” Gothic iddja “went,” Sanskrit e’ti “goes,” imas “we go,” ayanam “a going, way;” Avestan ae’iti “goes;” Old Persian aitiy “goes;” Lithuanian eiti “to go;” Old Church Slavonic iti “go;” Bulgarian ida “I go;” Russian idti “to go”). So called because ions move toward the electrode of opposite charge. n.
- An atom or a group of atoms that has acquired a net electric charge by gaining or losing one or more electrons.
- An atom or a group of atoms that has an electric charge. Positive ions, or cations, are formed by the loss of electrons; negative ions, or anions, are formed by the gain of electrons.
An atom that has either lost or gained one or more electrons, so that it has an electrical charge. Ions can be either positively or negatively charged.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary