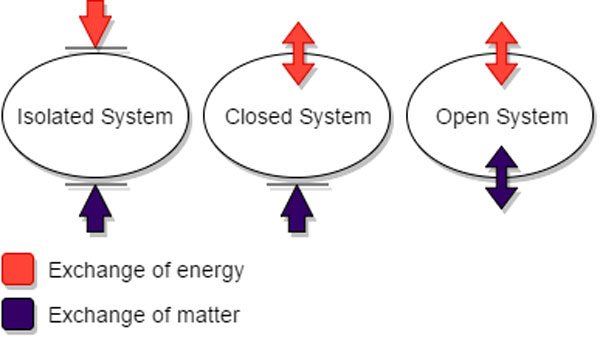
noun Thermodynamics.
- a region separated from its surroundings by a boundary that admits a transfer of matter or energy across it.
noun
- computing an operating system that is not specific to a particular supplier, but conforms to more widely compatible standards
- A physical system that interacts with other systems. The physical description of an open system can appear to violate conservation laws; for example, in a good description of the mechanism of energy transfer in a car engine (gears, driveshaft, and so on), energy will appear to be lost from the system over time, despite the law of conservation of energy. This is because the system is open, losing energy (in the form of heat) to surrounding systems (through friction). A system that loses energy in this way also called a dissipative system. Compare closed system.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary