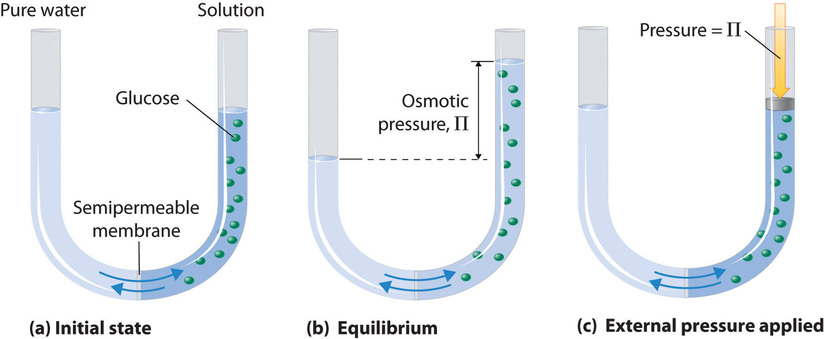
noun Physical Chemistry.
- the force that a dissolved substance exerts on a semipermeable membrane, through which it cannot penetrate, when separated by it from pure solvent.
noun
- the pressure necessary to prevent osmosis into a given solution when the solution is separated from the pure solvent by a semipermeable membrane
n.
- The pressure exerted by the flow of water through a semipermeable membrane separating two solutions with different concentrations of solute.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary