noun
- a form of oxygen, O3, with a peculiar odor suggesting that of weak chlorine, produced when an electric spark or ultraviolet light is passed through air or oxygen. It is found in the atmosphere in minute quantities, especially after a thunderstorm, is a powerful oxidizing agent, and is thus biologically corrosive. In the upper atmosphere, it absorbs ultraviolet rays, thereby preventing them from reaching the surface of the earth. It is used for bleaching, sterilizing water, etc.
noun
- a colourless gas with a chlorine-like odour, formed by an electric discharge in oxygen: a strong oxidizing agent, used in bleaching, sterilizing water, purifying air, etc. Formula: O 3; density: 2.14 kg/m³; melting pt: –192°C; boiling pt: –110.51°CTechnical name: trioxygen
- informal clean bracing air, as found at the seaside
n.1840, from German Ozon, coined in 1840 by German chemist Christian Friedrich Schönbein (1799-1868) from Greek ozon, neuter present participle of ozein “to smell” (see odor). So called for its pungent odor. n.
- A blue gaseous allotrope of oxygen formed naturally from diatomic oxygen by electric discharge or exposure to ultraviolet radiation. It is an unstable, powerfully bleaching, poisonous oxidizing agent with a pungent irritating odor, used to deodorize air, purify water, treat industrial wastes, and as a bleach.
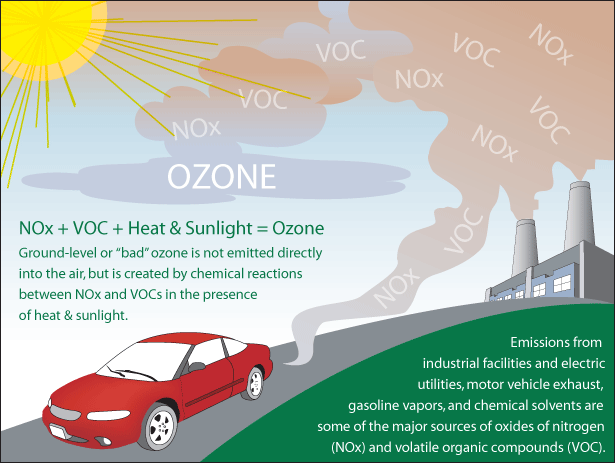
- An unstable, poisonous allotrope of oxygen having the chemical formula O3. Ozone forms in the atmosphere through the process of photolysis, when ultraviolet radiation from the Sun strikes oxygen molecules (O2), causing them to split apart. When freed oxygen atoms bump into and join other O2 molecules, they form ozone. Although ozone is broken down naturally in the atmosphere through chemical reactions with other atmospheric gases (such as nitrogen, hydrogen, and chlorine), in an unpolluted atmosphere the formation and breakdown of ozone is generally balanced, and the total concentration of ozone is relatively constant. The formation and destruction rates of ozone vary with altitude in the atmosphere, and with latitude. Most ozone forms in the 15 to 30 km (10 to 19 mi) altitude range and in latitudes closest to the equator where sunshine strikes the Earth the most. The ozone is then transported northward and southward by wind and is generally most concentrated in areas above the Canadian Arctic and Siberia and above Antarctica. Ozone is used commercially in water purification, in air conditioning, and as a bleach.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary