quantum number Word Origin noun Physics.
- any integer or half of an odd integer that distinguishes one of the discrete states of a quantum-mechanical system.
- any number that distinguishes among different members of a family of elementary particles.
Origin of quantum number First recorded in 1915–20 British Dictionary definitions for quantum number quantum number noun
- physics one of a set of integers or half-integers characterizing the energy states of a particle or system of particles. A function of the number multiplied by a fixed quantity gives the observed value of some specified physical quantity possessed by the system
quantum number in Science quantum number
- Any of a set of numbers that together fully determine the state of a quantum mechanical system by quantifying its individual properties. For example, four quantum numbers are used to specify the quantum state of an electron orbiting the nucleus of an atom: one characterizes its basic orbital energy level (principal or first quantum number), one the shape of its orbit (its azimuthal, orbital or second quantum number), one the orientation of its orbit relative to other orbits (magnetic or third quantum number), and one its spin (spin or fourth quantum number).
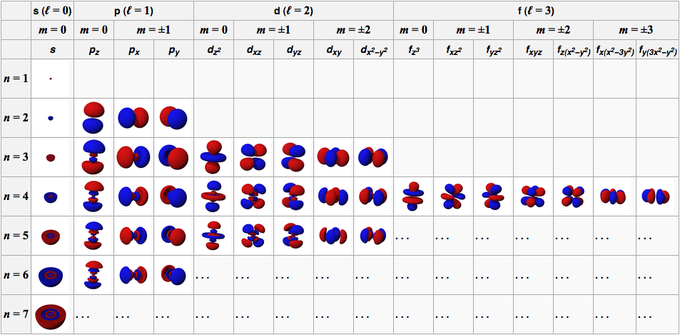
A Closer Look: Quantum numbers are used in quantum mechanics to describe the possible states of a physical system. Because many physical properties are quantized, taking on only discrete, distinct values, quantum numbers are generally integers or simple fractions, rather than continuous ranges. One of the great successes of quantum mechanics is its account of the structure of electron orbits around atomic nuclei, and the state of an electron in this particular system can be described using four quantum numbers. These are the principal or first quantum number, the orbital, azimuthal, or second quantum number, the magnetic quantum number, and the spin or spin magnetic quantum number. The principal quantum number, designated n, characterizes the basic energy level for the electron, and indicates in which shell the electron is located. It has integer values starting at 1; the higher the number, the farther the electron is from the atom’s nucleus. The principal quantum numbers correspond to the traditional orbital shell designations K, L, M, and so on, used in chemistry. The orbital quantum number, designated l, characterizes the electron’s angular momentum and determines the shape of it orbit. Its possible values for a given electron depend on the value of that electron’s principal quantum numbers, ranging from 0 to n-1. Because of these different possibilities, shells (other than the first shell) include subshells. These are traditionally designated s (where l=0), p (where l=1), d (where l=2), and f (where l=3). The magnetic quantum number, designated m or ml, takes on integer values between -l and +l, and indicates the orientation of the electron’s orbit within the subshell. For example, there are three orbitals in the p subshell, designated as px, py, and pz. Finally, the spin quantum number, designated ms, characterizes the spin direction of the electron. It can have values of + 12 or – 12. Electrons are fermions, meaning that no two electrons can be in the same quantum state (due to the Pauli exclusion principle); therefore, each electron in an atom is uniquely characterized by a set of these four quantum numbers. In fact, the chemical properties of atoms depend almost entirely on the quantum numbers associated with their electrons. Other quantum numbers are used to describe other physical systems, such as the shell structure of the atomic nucleus.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary