noun Chemistry.
- a chemically inert, radioactive gaseous element produced by the decay of radium: emissions produced by outgassing of rock, brick, etc. are a health hazard. Symbol: Rn; atomic number: 86; atomic weight: 222.
noun
- a colourless radioactive element of the rare gas group, the most stable isotope of which, radon-222, is a decay product of radium. It is used as an alpha particle source in radiotherapy. Symbol: Rn; atomic no: 86; half-life of 222 Rn: 3.82 days; valency: 0; density: 9.73 kg/m³; melting pt: –71°C; boiling pt: –61.7°C
heaviest gaseous element, 1918, from German Radon, from radium (q.v.) + -on suffix of inert gases. The element was identified in radioactive decay of radium. Alternative name niton (from Latin nitens “shining”) gained currency in France and Germany.
n. Symbol Rn
- A radioactive, largely inert gaseous element formed by the radioactive decay of radium and used as a radiation source in radiotherapy and research; its most stable isotope is Rn 222 with a half-life of 3.82 days. Atomic number 86.
Rn
- A colorless, odorless, radioactive element in the noble gas group. It is produced by the radioactive decay of radium and occurs in minute amounts in soil, rocks, and the air near the ground. Radon is used as a source of radiation for the treatment of cancer and other diseases. Its most stable isotope is Rn 222 with a half-life of 3.82 days. Atomic number 86; melting point -71°C; boiling point -61.8°C; specific gravity (solid) 4. See Periodic Table.
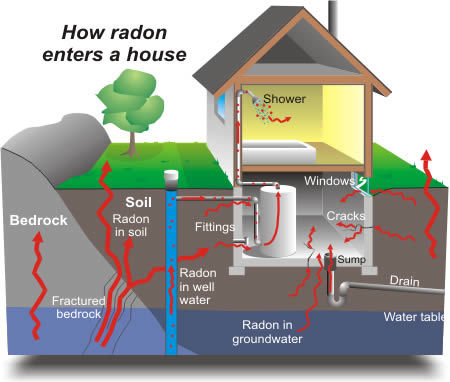
A colorless, odorless, radioactive gas that is produced by the decay of radium in the soil.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary