noun Physical Chemistry.
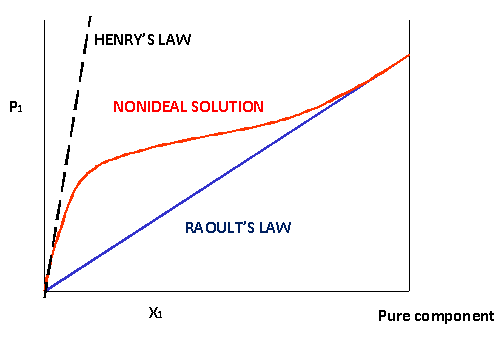
- the principle that the fraction by which the vapor pressure of a solvent is lowered by the addition of a nonvolatile, nonelectrolytic solute is equal to the mole fraction of the solute in the solution.
n.
- The principle that the vapor pressure of a solution is equal to the vapor pressure of the pure solvent multiplied by the mole fraction of the solvent in the solution.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary