noun Thermodynamics.
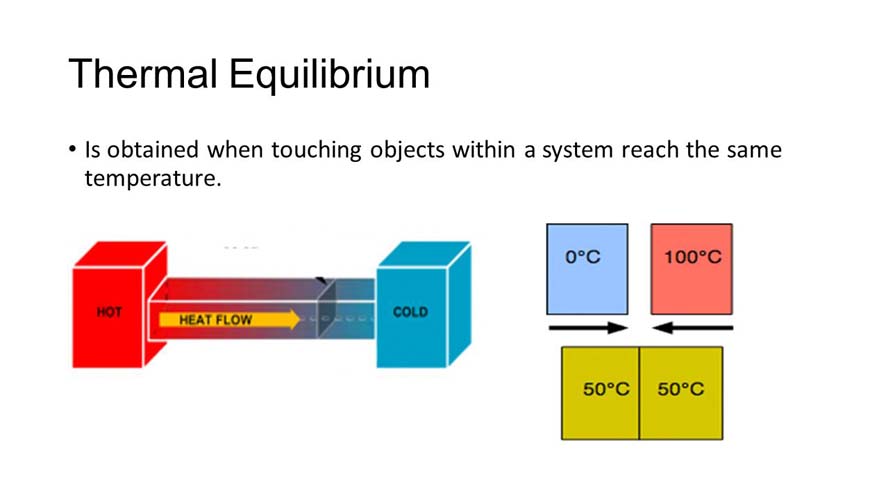
- the relationship between two systems connected only by a diathermic wall.
- the relationship between two isolated systems the states of which are such that no net transfer of energy would occur between them if they were connected by a diathermic wall.
- The condition under which two substances in physical contact with each other exchange no heat energy. Two substances in thermal equilibrium are said to be at the same temperature. See also thermodynamics.
In physics and chemistry, a condition in which all parts of a system are at the same temperature.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary