verb (used with or without object), ti·trat·ed, ti·trat·ing. Chemistry.
- to ascertain the quantity of a given constituent by adding a liquid reagent of known strength and measuring the volume necessary to convert the constituent to another form.
noun
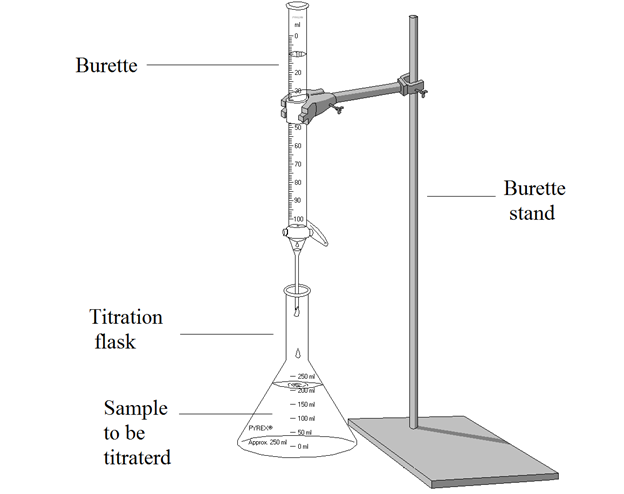
- an operation, used in volumetric analysis, in which a measured amount of one solution is added to a known quantity of another solution until the reaction between the two is complete. If the concentration of one solution is known, that of the other can be calculated
verb
- (tr) to measure the volume or concentration of (a solution) by titration
n.1864, from French titrer, from titre “standard, title” (see title (n.)), also “fineness of alloyed gold;” in chemistry, the establishment of a standard strength or degree of concentration of a solution. v.1870, from French titrer, from titre “title, qualification” (see titration). n.
- The process, operation, or method of determining the concentration of a substance in a solution to which the addition of a reagent having a known concentration is made in carefully measured amounts until a reaction of definite and known proportion is completed, as shown by a color change or by electrical measurement, and then calculating the unknown concentration.
v.
- To determine the concentration of a solution by titration or perform the operation of titration.
- The process or operation of determining the concentration of a substance in solution. Titration is performed by adding to a known volume of the solution a standard reagent of known concentration in carefully measured amounts until a reaction of definite and known proportion is completed (as shown by a color change or by electrical measurement) and then calculating the unknown concentration.
In chemistry, the determination of what materials are present in a sample by adding precise amounts of known chemicals and observing the chemical reaction.
 Liberal Dictionary English Dictionary
Liberal Dictionary English Dictionary